Pvt Relationship In Thermodynamics
He has been teaching undergraduate and graduate core subject courses at the University of California, Berkeley (UC Berkeley), USA, since.
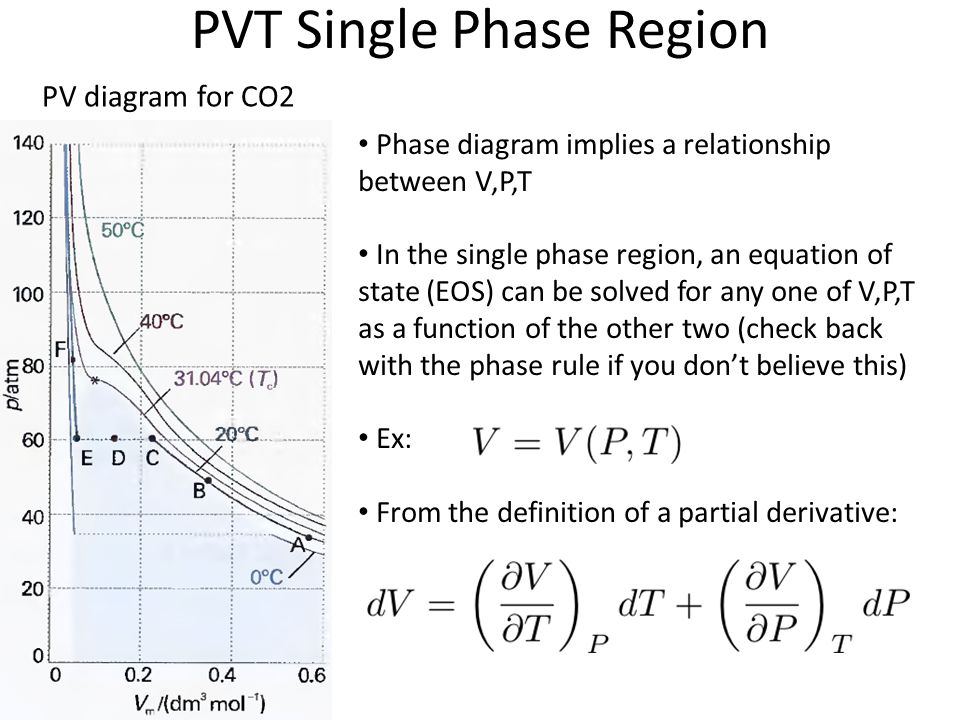
Pvt relationship in thermodynamics. The equation of state for an ideal Bose gas is. Being able to decode the information shown in a PV diagram allows us to make statements about the change in internal energy. Note the relationship between Q 12 and W 12 determined from an energy balance during step 1-2.
Additional information on equations of state are given in the article PVT Relationships. Equating the two first law expressions given above. Potentiometric measurements are based on the Nernst equation, which was developed from thermodynamic relationships and is therefore valid only under equilibrium (read thermodynamic) conditions.
Equations, PdV-Work, Heat, Pressure and Temperature Measurement. DU = dq +dW From the second law:. Note that dU = m C V dT, C V = (R/MW)/(g-1), PV = nRT and dW = P dV.
And we were in a state of equilibrium. The enthalpy ( H), internal energy ( U), and heat capacity ( C p) used in evaluating energy effects of processes via energy balances;. 6-2 FUNDAMENTAL THERMODYNAMIC RELATIONSHIPS FOR PURE COMPONENTS.
7E-3 - Work and ΔS for IGs Undergoing Isothermal, Polytropic and Adiabatic Processes;. As with everything oil and gas related, the amount of time it takes to extract the hydrocarbons from the subsurface geology increases the cost of the well. It was first stated by Benoît Paul Émile Clapeyron in 14 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law.
PVT Diagram in Hindi Thermodynamics Tutorial by D Verma Sir - Duration:. PVT V T p V T pV a a a a a a = = = 2. As a gas goes through a thermodynamics process, the state of the gas will shift around in the PV diagram, tracing out a path as it moves (as shown in the diagram below).
Compare h obtained this way with the Carnot cycle efficicency and you will arrive at the desired conclusion. 11-1 The Virial Equation of State:. Why do you mean by saying "When we impose the restraint of constant pressure, why don't the quantities (∂S/∂T)V and (∂S/∂V)T somehow change?" in my opinion, the second last equation means that if we fix the pressure and change the temperature, the rate of entropy change with respect to temperature change equals to the rate of.
(b) Just use the result from part (c ) along with the ideal gas equation to convert V to P. Heat and Work are both forms of Energy. APPLIED THERMODYNAMICS TUTORIAL 2 GAS COMPRESSORS In order to complete this tutorial you should be familiar with gas laws and polytropic.
Thermometers and Measurement of Temperature. Ideal Bose equation of state. 11-3 Other Equations of State:.
First law of thermodynamics furnishes the relationship between (a) heat and work (b) heat, work and properties of the system (c) various properties of the system (d) various thermodynamic processes (e) heat and internal energy. PvT Surface for a Substance which Contracts Upon Freezing The equilibrium states of a simple, compressible substance can be specified in terms of its pressure, volume and temperature. In order to carry through a program of finding the changes in the various thermodynamic functions that accompany reactions—such as entropy, enthalpy, and free energy—it is often useful to know these quantities separately for each of the materials entering into the reaction.
I hope that students, faculty and interested observers will share their thoughts on the laws of thermodynamics, phase and chemical equilibrium and many related topics. Thermodynamics, science of the relationship between heat, work, temperature, and energy. Summary.Climate scientists promoting greenhouse gas theories usually omit or dismiss consideration of thermodynamics and rely on empirical models and observed data to assess the effect of anthropogenic CO2 (carbon dioxide) from combustion of ‘fossil fuels’ on the global and surface temperatures of the Earth.
Thermodynamics - Thermodynamics - Thermodynamic properties and relations:. Suggested Readings and References:. They are however can vibrate about this fixed position.
O Internal energy o Entropy These are not measurable and required for mathematical formulations and understanding of thermodynamic laws. You will arrive at the form (1/T) dT and (1/V) dV on both sides. Thermodynamic Properties 3.1 Phase and Pure Substance A phase is a quantity of matter characterized by both uniform physical structure and uniform chemical composition.
This page uses frames, but your browser doesn't support them. Equations are given that related the thermodynamic properties to ionic strength and salinity. For calculating V (To,P)/V (To,0), the P-V relation at constant To is developed using the Chopeias- Boehler approximation 7, 8 with the help of the Anderson-Gruneisen parameter definition.
Pressure Temperature and Volume Gas Law Relationship - Duration:. Saturated and subcritical regions. The pressure exerted by a gas in sealed container is 100kPa at 17 o C.
Ch 8 - Thermodynamics of Flow Processes:. Back to Top of this Page. So a process in which at each moment the system is in thermodynamic equilibrium with the surrounding is known as a quasi-static process.
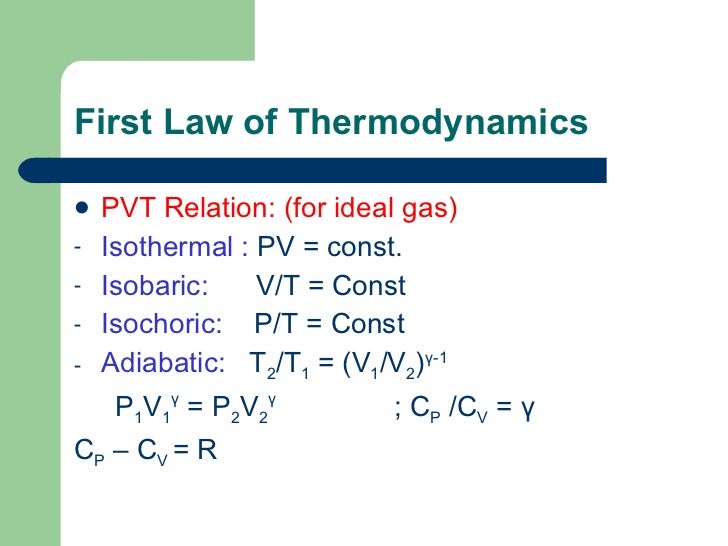
Thermodynamics the study of the transformations of energy from one form into another First Law:. C p - c v = R. {\displaystyle pV_ {m}=RT~ {\frac { {\text {Li}}_ {\alpha +1} (z)} {\zeta (\alpha )}}\left ( {\frac {T} {T_ {c}}}\right)^ {\alpha }}.
Thermodynamics deals with the large scale response of a system which we can observe and measure in experiments. Thermodynamic properties of unsaturated vapour and liquid states from a cubic equation of state:. PVT relationship for liquids A generalized correlation to estimate the molar volume of saturated liquid proposed by Rackett (1).
The first law of thermodynamics with Q=0 shows that all the change in internal energy is in the form of work done. 112 Fundamental thermodynamic properties Those appear from fundamental laws of thermodynamics. As mentioned above, the Nernst equation relates potential to the concentration of electroactive species.
This condition can be used to derive the expression for the work done during an. C p dT - vdp = c v dT + pdv (c p - c v)dT = d(pv) c p - c v = d(pv)/dT and pv = RT. T dq dS ≥ Where, for irreversible system T dq dS > and, for reversible system dq dS = T For a closed system in which only reversible pV work is involved dW = −pdV and T dq dS =.
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. So before I did anything, when my canister was just here, I had all the pebbles on it. In this article we will discuss about how to measure work, heat, pressure and temperature.
$\begingroup$ I can't fully understand your question too. Relationships between thermodynamic properties c v, c p, and R. This led to the devising of a new thermodynamic absolute temperature scale or Kelvin scale which starts at OK.
Thermodynamics is all about the relationship between temperature and energy. As aerodynamicists, we are most interested in the thermodynamics of propulsion systems and high speed flows.The propulsion system of an aircraft generates thrust by accelerating a working fluid. It has been discussed that state variables are defined only when the thermodynamic system is in equilibrium with the surrounding.
Pressure volume temperature (PVT) analysis is the process of determining the fluid behaviors and properties of oil and gas samples from an existing well. Integrate to obtain the desired result. Saturday, April 01, 06 HW #2 - P #5 - PVT Relationship Applied to an Automobile Tire.
Process, PVT Relationship, PV diagram, TS diagram, Change in Internal Energy, Change in. P V m = R T Li α + 1 ( z ) ζ ( α ) ( T T c ) α. Only the existence of thermodynamic equations of state follows from the first law of thermodynamics, but the relationship between the equations of state and thermodynamic equations of state (∂ U /∂ V) T = T (∂ p /∂ T) V – P follows from the second law of thermodynamics.
CYCLE FOR RECIPROCATING COMPRESSOR 2.1 THEORETICAL CYCLE The diagram shows the basic design of a reciprocating compressor. Thermodynamics is a branch of physics which deals with the energy and work of a system. These include the PVT relationship of the EoS for volumetric behavior;.
THERMODYNAMICS CONTENTS INTRODUCTION 6 Introduction, Classification of Thermodynamics System, Closed System, Open System, Isolated System, Basic Terminology, Thermodynamic Equilibrium, Control Volume, Steady State,. Note that dU = m C V dT, C V = (R/MW)/ ( g -1), PV = nRT and dW = P dV. I could describe all of its macrostates, its pressure, its volume, its temperature.
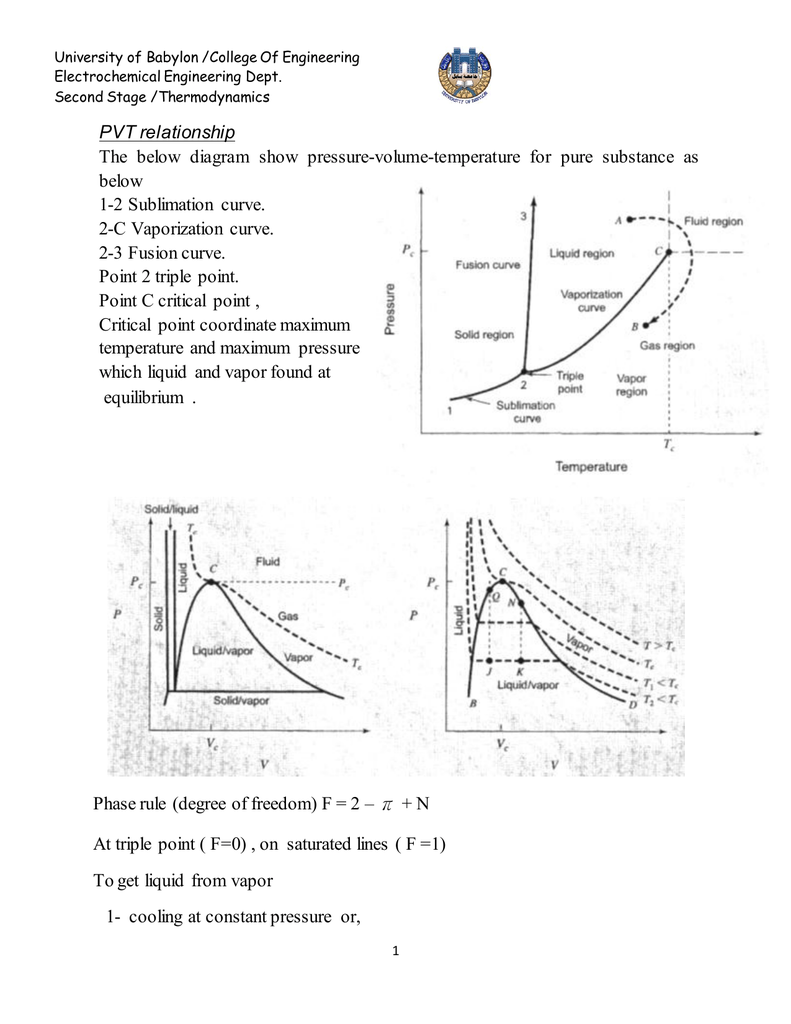
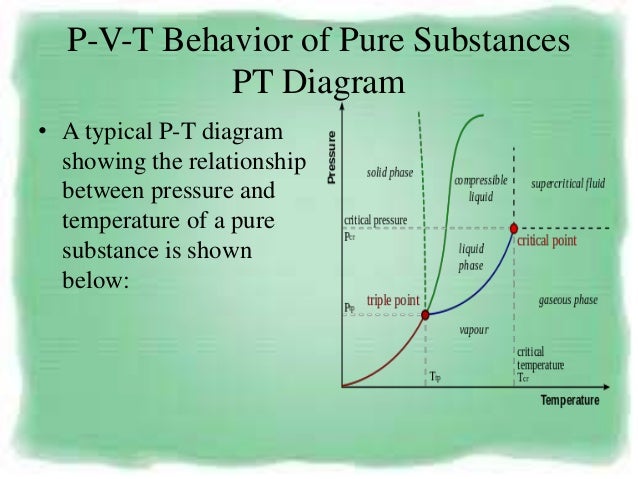
P-T Diagram • The three lines 1-2, 2-3 and 2-C display conditions of P and T at which two phases may co-exist in equilibrium, and are boundaries for the single-phase regions of solid, liquid and vapor (gas). The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work. G= c p /c v.
Change in enthalpy in a closed system is equal to heat transferred if the reversible process takes place. 7E-5 - Power Input for an Internally Reversible, Polytropic Compressor;. And this will give you an idea, or start giving you an idea of why people who study thermodynamics love these so much.
In any process, Energy can be changed from one form to another (including heat and work), but it is never created or distroyed:. A phase can be solid, liquid, vapor or gas. Thermochimica Acta 1992, 196 (2) , 415-435.
From this relationship we can arrive at relationships for several other types of thermodynamic process:. Posted Apr 12, 16. The entropy ( S) used in evaluating the properties of.
Tabular and graphical presentations of the p-v-T relationship, analytical formulations, called equation of state, constitute another way of expressing the p-v-T relationship. The first law of thermodynamics governs changes in the state function we have called internal energy (\(U\)). Changes in the internal energy (ΔU) are closely related to changes in the enthalpy (ΔH), which is a measure of the heat flow between a system and its surroundings at.
Newman is considered one of the great chemical engineers of his time. This puts a constraint on the heat engine process leading to the adiabatic condition shown below. Thermodynamics deals with the transfer of energy from one place to another and from one form to another.
Conservation of Energy. As p, v, and T are measurable properties so equations of state are commonly known as pvT relationships. Adiabatic Process An adiabatic process is one in which no heat is gained or lost by the system.
It was found that the container might leak if the. When , the process is isobaric When , the process is isothermal When , the process is isentropic When , the process is isochoric Reversible. If any two of these state variables is specified, the third is determined.
Equations for Work Done in Various Processes 3. The branch of science that deals with the study of different forms of energy and the quantitative relationships between th. Ice melts at 0 o C or 273K and water boils at 100 o C or 373K.
To understand the relationship between internal energy and entropy. Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube. The atoms in a solid phase are fixed relative to other atoms in the solid.
His reputation derives from his mastery of all phases of the subject matter, his clarity of thought, and his ability to reduce complex problems to their essential core elements. This is because thermodynamics deals with how heat and temperature are related to energy. 7E-2 - PVT Relationships for Isentropic, IG Processes;.
11-2 Two-Constant Equations of State:. PvT BEHAVIOR OF REAL GASES AND REAL-GAS MIXTURES:. Thermodynamics provides relationships among many useful properties.
P-V-T Behavior of Pure Substances PT Diagram • A typical P-T diagram showing the relationship between pressure and temperature of a pure substance is shown below:. The thermodynamic relationship allows the determination of the thermic and caloric equations of state, if the thermodynamic potential, specified in the form of a function of appropriate variables, is known. Relationship between Pressure (P), Volume (V), Temperature (T) and quantity;.
7E-4 - Performance of an Ideal Gas Cycle;. The thermodynamic properties of average seawater have been summarized based on the recent measurements of the PVT properties. Your Brain and the Second Law of Thermodynamics An entropic mind is a terrible thing to waste.
A reversible process is one which is performed as if it were always at equilibrium, and without the production on entropy. Fundamental equations of Thermodynamics (1) The combined first and second law From the first law:. Using the principles of thermodynamics, we can keep people comfortable in a building while using energy in smarter ways.
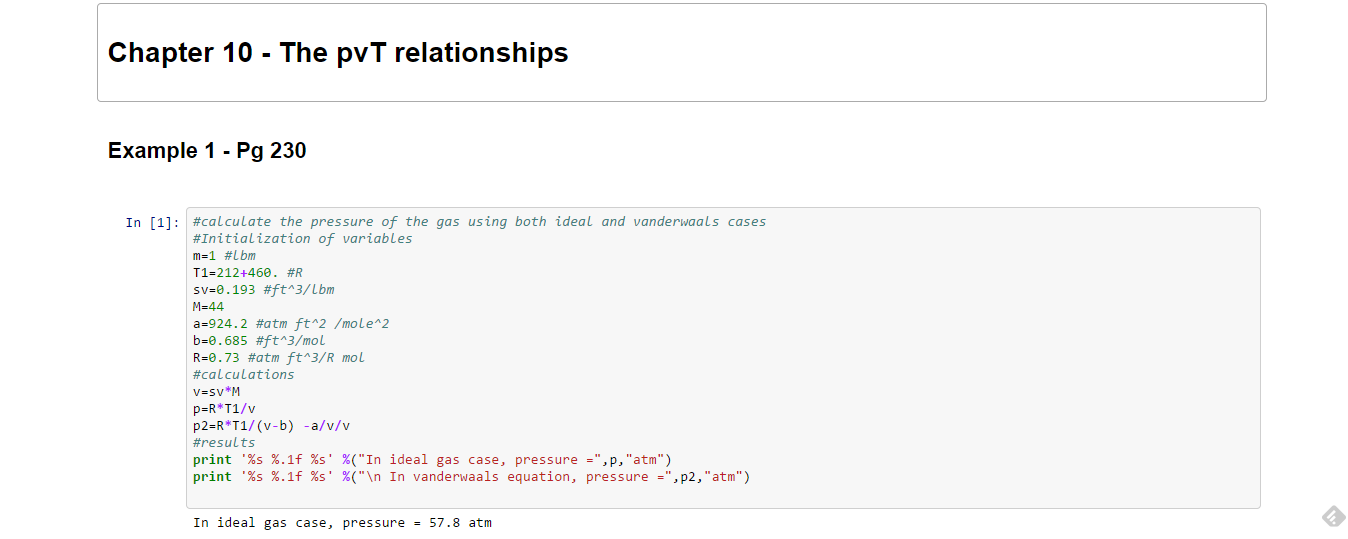
Relation between PVT Gas Laws:. (c) Easier to do part (c) before (b). Examples of P–V–T calculations.
For estimating the compression V (T,P)/V (Ty,0), we need to compute the values of V (To,P)/V (To,0) and V (T.P)/V (To,P) separately, where To is the initial temperature. Apply the 1st Law to step 2-3. Mechanical and Thermodynamic Work 2.
A PVT relationship is one of the forms of the equations of state (see Equations of State), which relates the pressure, molar volume V and the temperature T of physically homogeneous media in thermodynamic equilibrium. To form equations of state for a solid body.
Derivation Of Thermodynamic Equations Ppt Video Online Download

Pdf Microsoft Excel Spreadsheets For Calculation Of P V T Relations And Thermodynamic Properties From Equations Of State Of Mgo Diamond And Nine Metals As Pressure Markers In High Pressure And High Temperature Experiments
2
Pvt Relationship In Thermodynamics のギャラリー

Pressure Volume Temperature Relationship Of Pure Fluids Ppt Download

Thermodynamic Surface An Overview Sciencedirect Topics

1 Solve The Items Of Applied Chemical Thermodynamics Determine A Relationship Between Cp And The Homeworklib
Www Kau Edu Sa Files Files 119 Handouts 6 Pdf
2

Adiabatic Process Relation Between P V And T Testbook
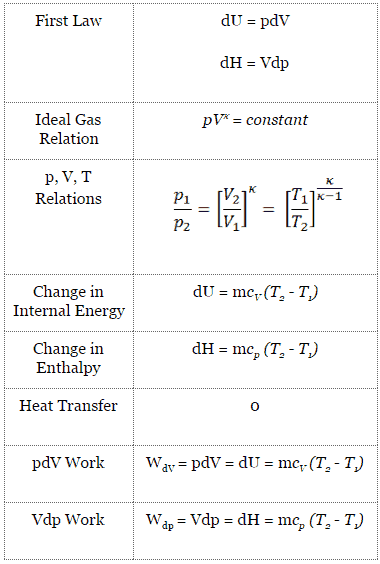
What Is Adiabatic Process Definition
Search Q Pvt Relationship For Ideal Gas Tbm Isch
Python Textbook Companion Project Fossee Iit Bombay
Http Www Sfu Ca Mbahrami Mech 240 Lectures Summary thermodynamics problems Pdf

Chapter 2b Pure Substances Ideal Gas Updated 1 17 11
Adiabatic Pvt Relationships Why Kelvin And Not Celsius Physics Forums

Proof Of Pressure Volume And Temperature Ratio Adiabatic Process Youtube
Ceng Tu Edu Iq Ched Images Lectures Chem Lec St3 C4 Lecture 12 Pdf

Ch Thermodynamics By Career Avenues Issuu
University Of Babylon College Of Engineering Electrochemical Engineering Dept Second Stage Thermodynamics
Search Q Adiabatic Process Formula Tbm Isch
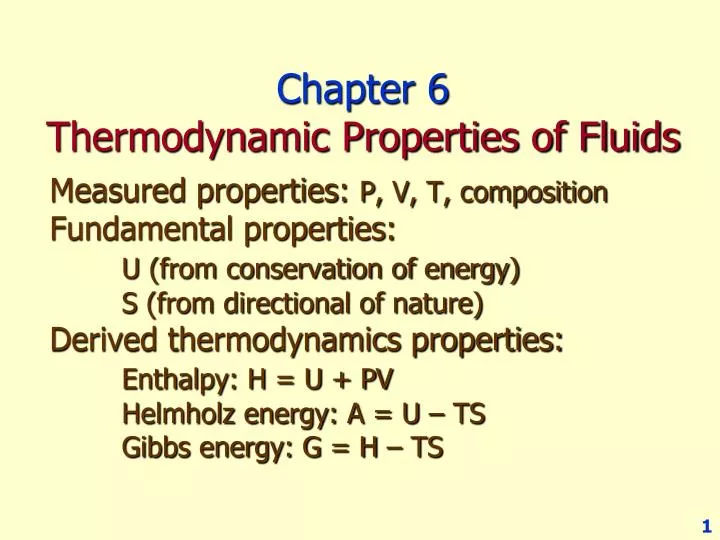
Ppt Chapter 6 Thermodynamic Properties Of Fluids Powerpoint Presentation Id

Maxwell S Thermodynamic Surface Wikipedia

Part 2 Thermodynamic Properties Pvt Data And Thermal Properties Subvolume A Polymer Solids And Polymer Melts Landolt Bornstein Numerical Data In Science And Technology New Series 6a2 Pionteck J

Ideal And Real Gases Thermodynamic Relations
State Of Working Fluid
Http Ocw Utm My Mod Resource View Php Id 919
Pvt Behaviour Of Gases And Relations

Pptx Thermodynamic Applied And Interdisciplinary Physics Continuum Mechanics
Http Purl Fcla Edu Fcla Etd Ufe
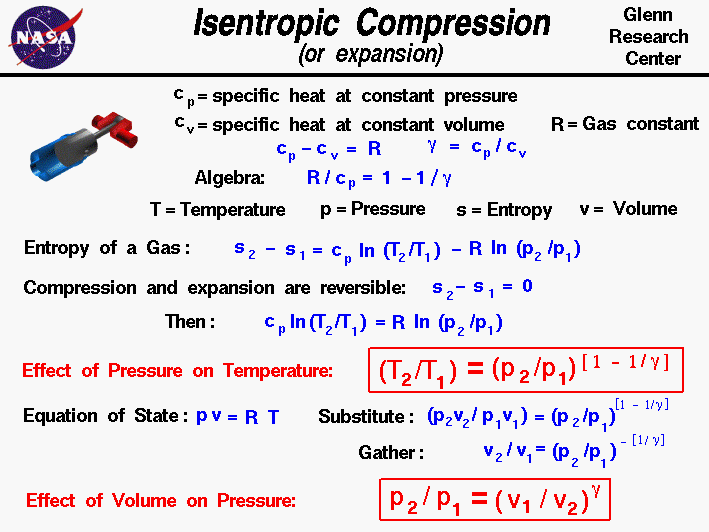
Isentropic Compression Or Expansion
Revision On Thermodynamics
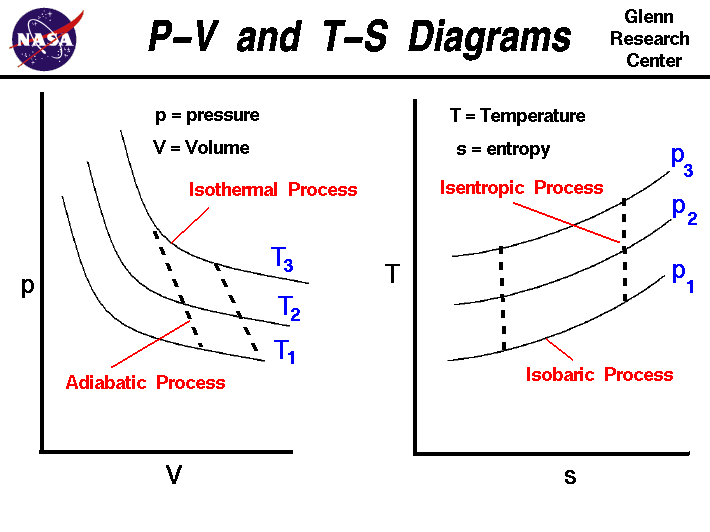
P V And T S Diagrams

Cyclic Relation Among With Thermodynamic Properties Like P V And T Youtube

P V T Surface In Thermodynamics Mechanical Engineering Concepts And Principles

Adiabatic Process P V T Relation Youtube
Http Citeseerx Ist Psu Edu Viewdoc Download Doi 10 1 1 710 59 Rep Rep1 Type Pdf
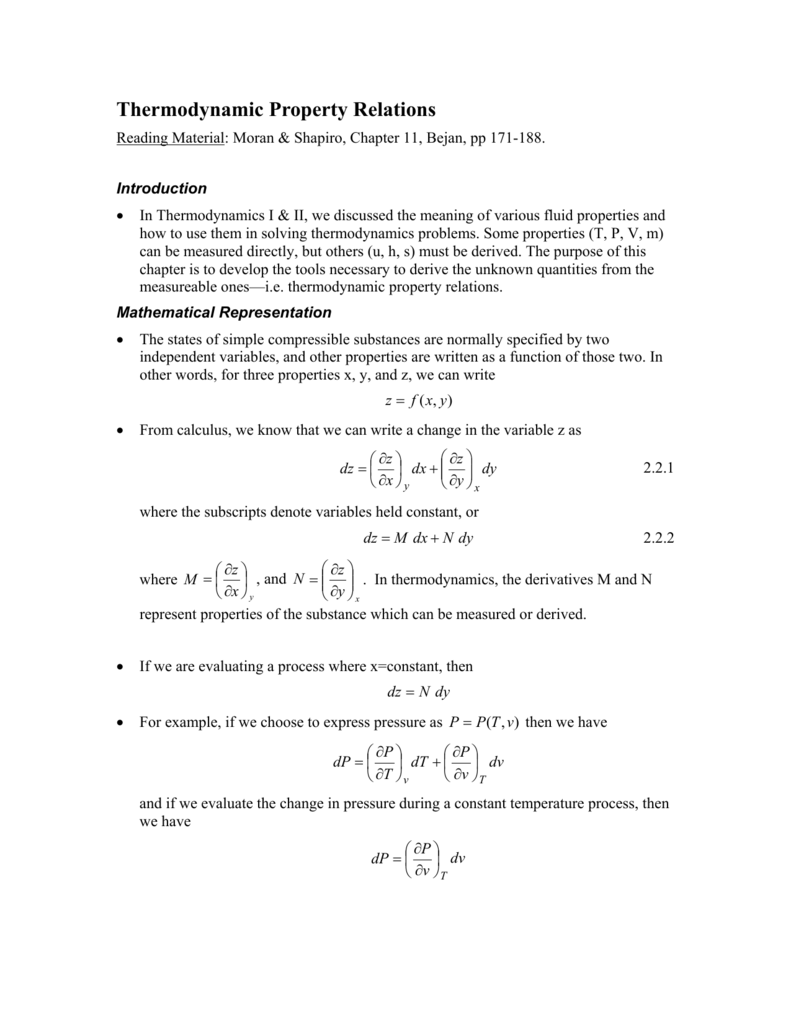
Thermodynamic Property Relations
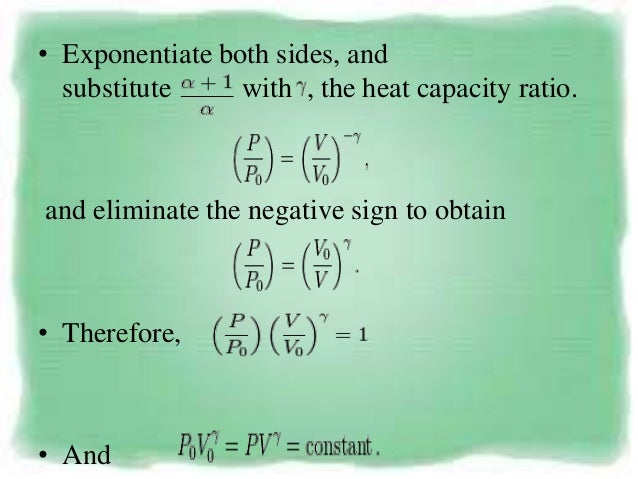
Pvt Behaviour Of Gases And Relations
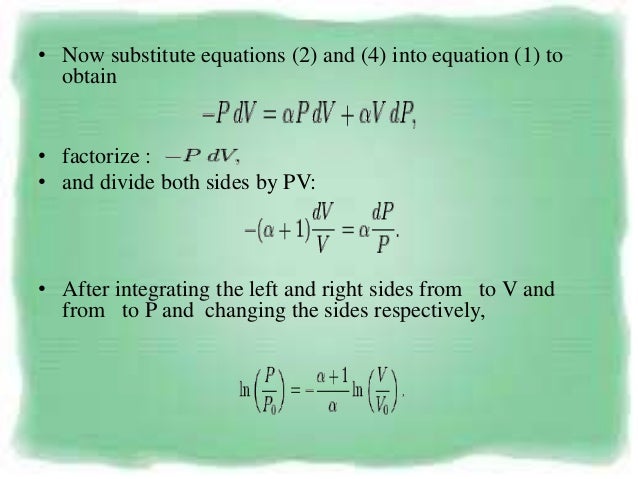
Pvt Behaviour Of Gases And Relations
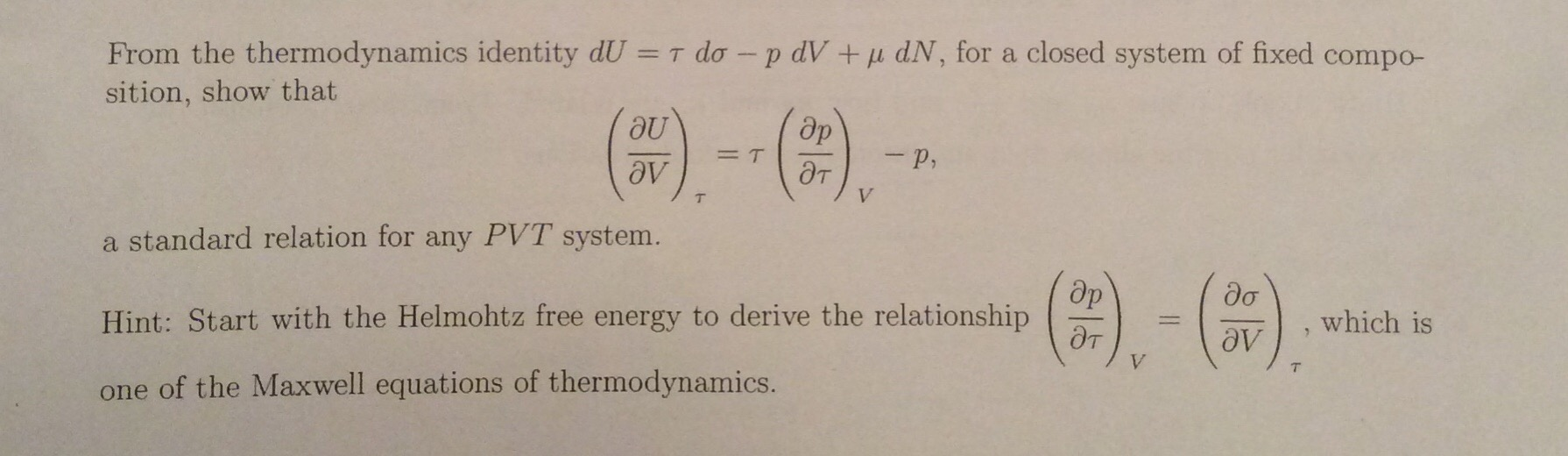
Solved From The Thermodynamics Identity Du T Do P Dv Chegg Com
Http Pu Edu Pk Images Image Lecture Notes Advanced Chemical Engineering Thermodynamics Pdf

Isentropic Process Nuclear Power
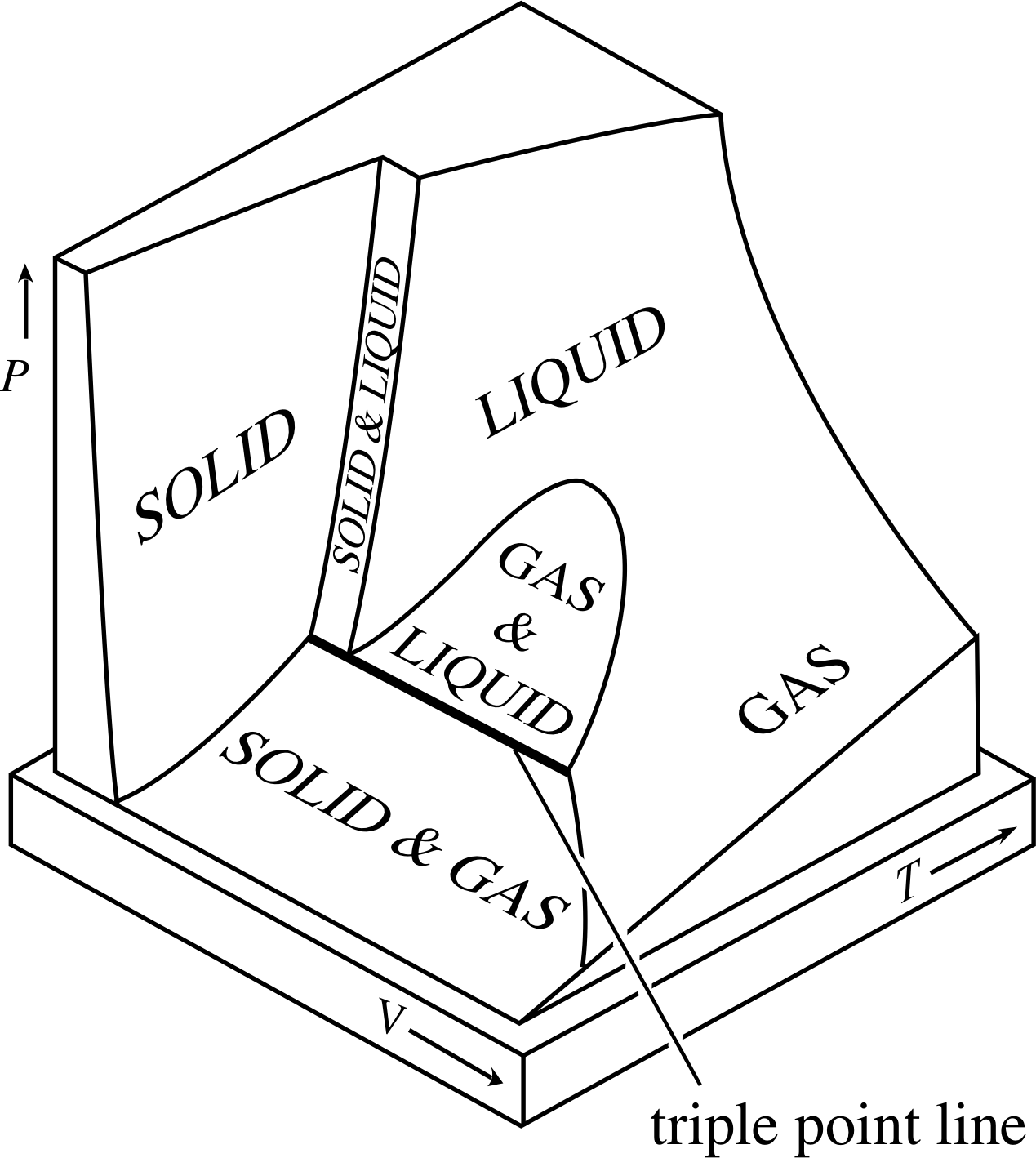
Pplato Flap Phys 7 3 Internal Energy Heat And Energy Transfer

1 The Ideal Gas 2 Ideal Gas Equation Of State Property Tables Provide Very Accurate Information About The Properties It Is Desirable To Have Simple Ppt Download

Perry S Chemical Engeneers Handbook Parte 5 Thermodynamics Docsity
Http Ska10 Weebly Com Uploads 4 7 1 1 Unit Iv Pdf
Equation Of State For Non Ideal Gases

Thermodynamic Relationship An Overview Sciencedirect Topics
Courses Physics Ucsd Edu 18 Spring Physics210a Lectures Ch02 Pdf

Develop Separate Excel Vba Based Numerical Density Chegg Com
2

Engineering Thermodynamics Lecture Notes

The Deviation Rate Of The Second Law Of Thermodynamics Is 14 6

Chapter 3 Thermodynamics

Thermodynamics And Equations Of State Of Iron To 350 Gpa And 6000 K Topic Of Research Paper In Earth And Related Environmental Sciences Download Scholarly Article Pdf And Read For Free

Equation Of State Wikipedia

Polytropic Processes For An Ideal Gas Youtube

Doc Cctd101b Engineering Thermodynamics Tutorial 2 Properties Pvt Relationships Joel P Academia Edu
Thermal Engineering Relation Between Pvt

First Law Of Thermodynamics Internal Energy Video Khan Academy

Equation Of State Wikipedia
2
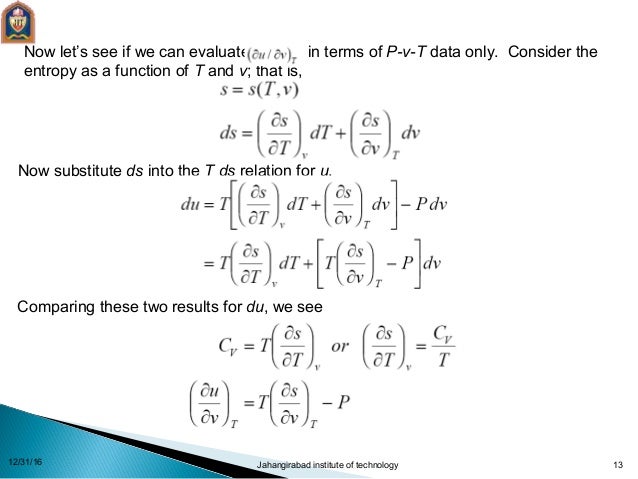
Thermodynamics Relations

Modification Proposed For Srk Equation Of State Oil Gas Journal

Pdf Chapter 18 Heat And The First Law Of Thermodynamics Conceptual Problems Ana Claudia Academia Edu

Eos
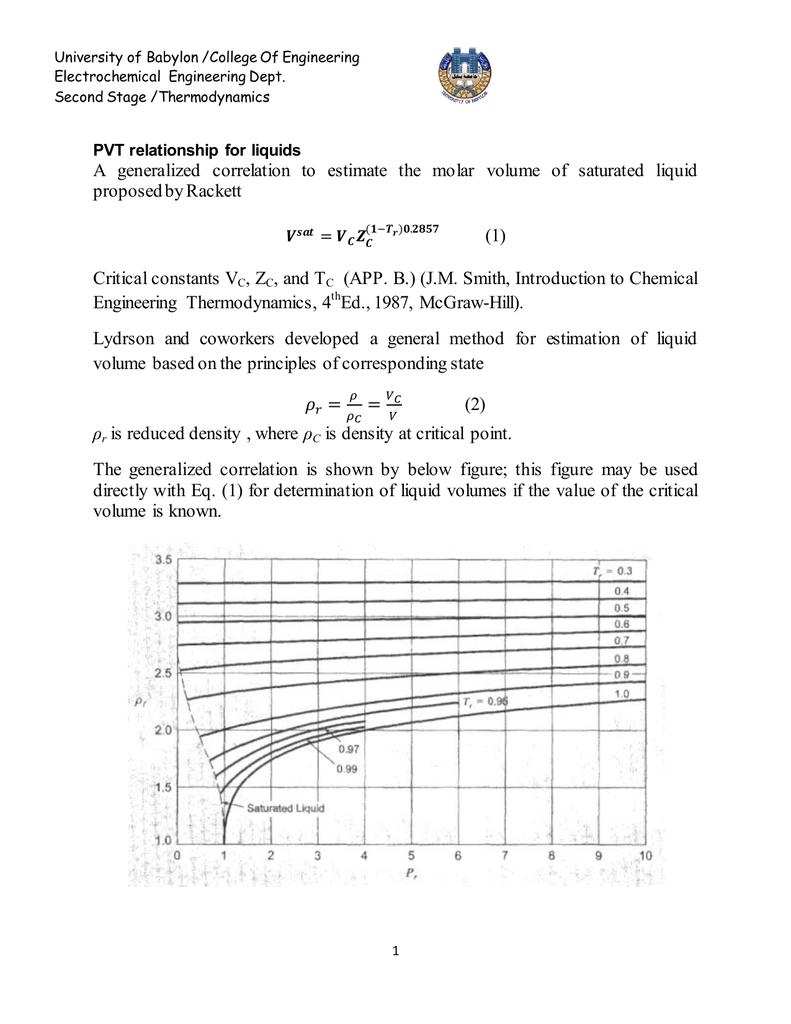
University Of Babylon College Of Engineering Electrochemical Engineering Dept

Graphical Representations Of The Equation Of State Of Water Thermodynamics
Introducing Thermodynamics Through Energy And Entropy Thermodynamics A Brief Introduction

Ch Thermodynamics By Career Avenues Issuu
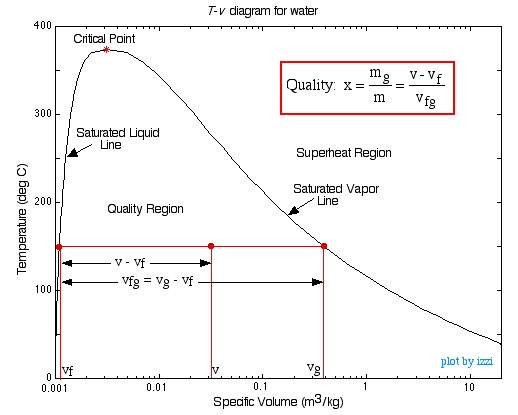
Chapter 3 Thermodynamics

13 Thermodynamics Proof Of Adiabatic Equation Most Important Complete Concept Youtube

Game View Of 3d P V T Surface For Revised Thermo Game Download Scientific Diagram
Q Tbn 3aand9gcsfmimi Kllwngkchdng0qjdulspbw Siup Hvqxb9ok1hee1hl Usqp Cau

How To Do P V T Pressure Volume Temperature Gas Calculations Formula Boyle S Law Charles S Law Gay Lussac S Law Ideal Gas Behaviour Problem Solving Revision Examples Graphs Gcse Chemistry Physics Ks4 Science A
Http Pu Edu Pk Images Image Lecture Notes Advanced Chemical Engineering Thermodynamics Pdf

Thermodynamic Properties Property Table W Property Table From Direct Measurement W Equation Of State W Equation Of State Any Equations That Relates Ppt Download
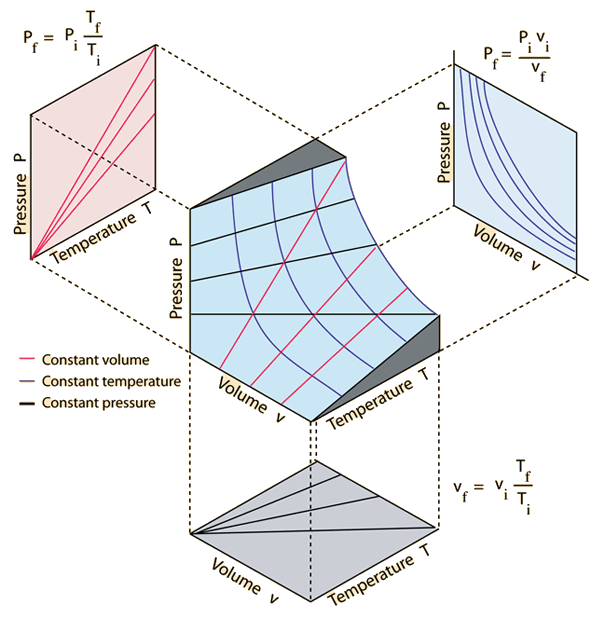
Ideal Gas Law

Eos

Chapter 5 A A A Single Phase Systems A Aˆa

How To Do P V T Pressure Volume Temperature Gas Calculations Formula Boyle S Law Charles S Law Gay Lussac S Law Ideal Gas Behaviour Problem Solving Revision Examples Graphs Gcse Chemistry Physics Ks4 Science A
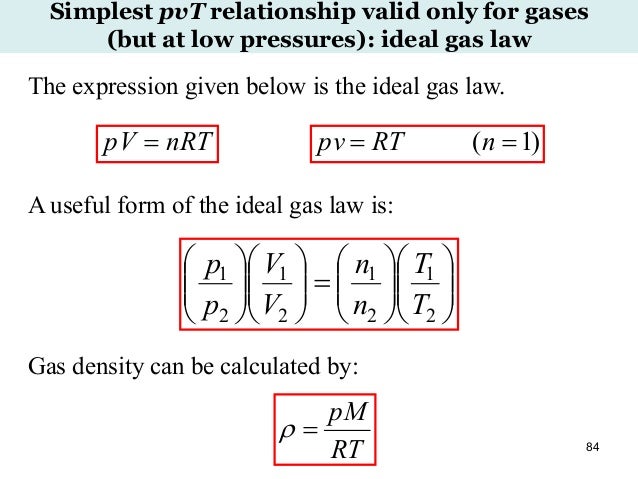
Advanced Chemical Engineering Thermodynamics 31 July 16
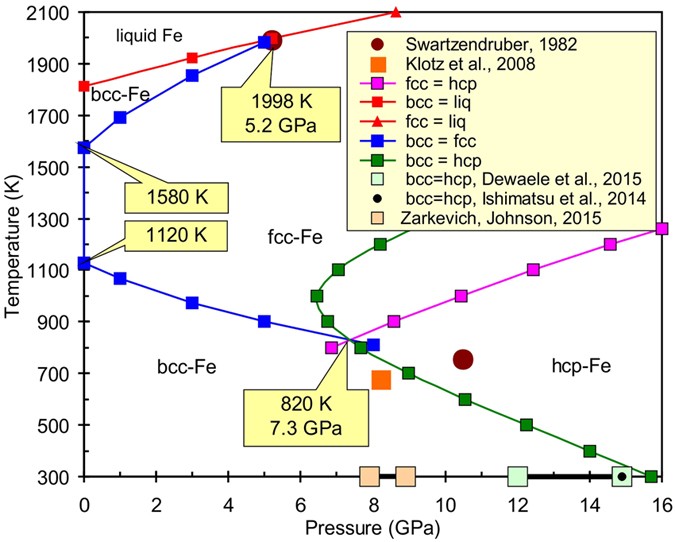
Thermodynamics And Equations Of State Of Iron To 350 Gpa And 6000 K Scientific Reports

Chapter 2b Pure Substances Ideal Gas Updated 1 17 11
Q Tbn 3aand9gcsbqymjkje7talolf27asvtgtvyabbo2wxz C1qsp8chlsqykes Usqp Cau
Does Evolution Contradict The Second Law Of Thermodynamics

Chemical Engineering Thermodynamics I Heat Gases

Engineering Thermodynamics K Mohanraj Nag P K Engineering Thermodynamics Tata Mcgraw Hill Pdf Document
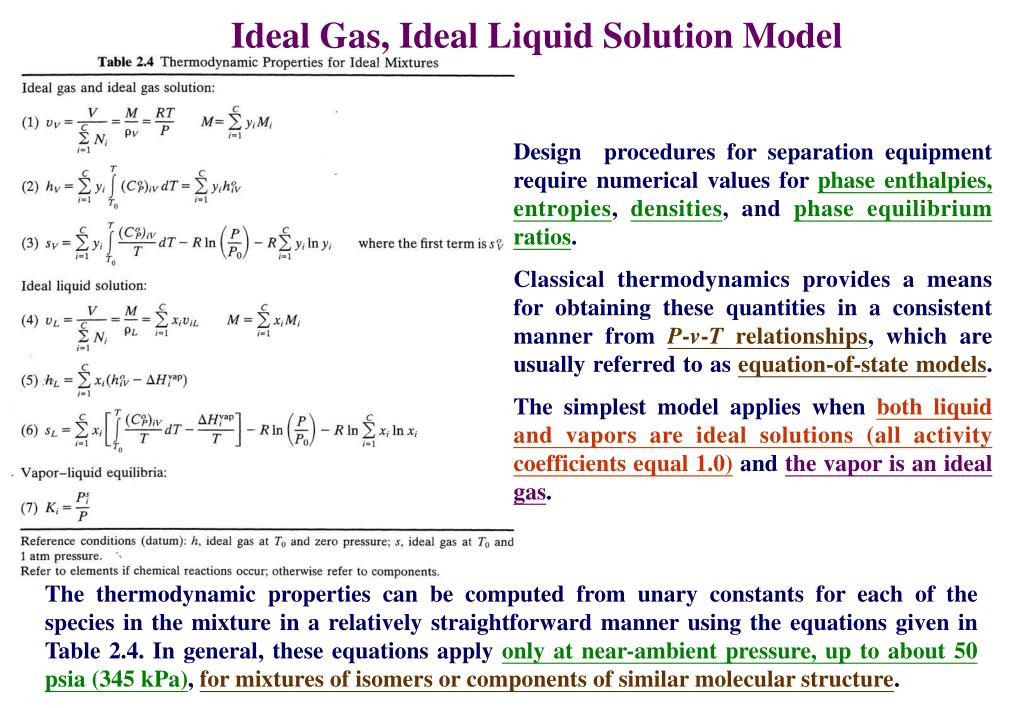
Ppt Thermodynamics Of Separation Process Powerpoint Presentation Free Download Id

Experimental Thermodynamics Volume Ii Springerlink

A New Approach For Estimation Of Pvt Properties Of Pure Gases Based On Artificial Neural Network Model
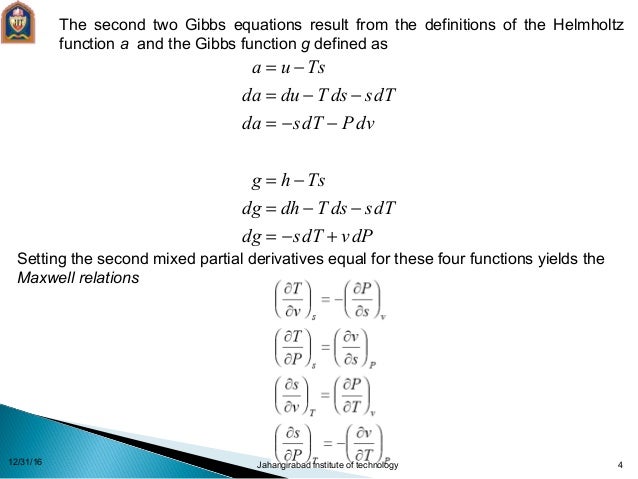
Thermodynamics Relations

Use Of The Velocity Of Sound In Predicting The Pvt Relations Sciencedirect
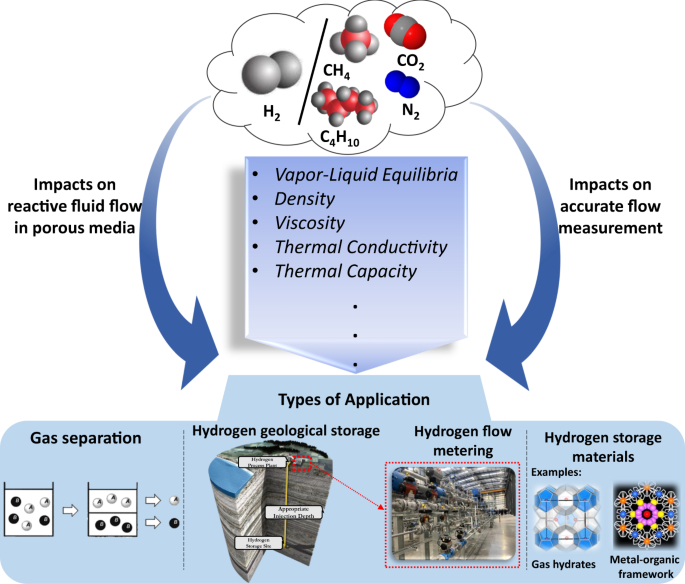
Thermodynamic And Transport Properties Of Hydrogen Containing Streams Scientific Data

Adiabatic Process Relation Between P V And T Testbook

Pdf Thermodynamic Property Relations Lono Satudelapan Academia Edu
공정 열역학 Chapter 6 Thermodynamic Properties Of Fluids Ppt Download

Critical Point Thermodynamics Wikipedia
Www Osti Gov Servlets Purl

Chapter 3 Thermodynamics
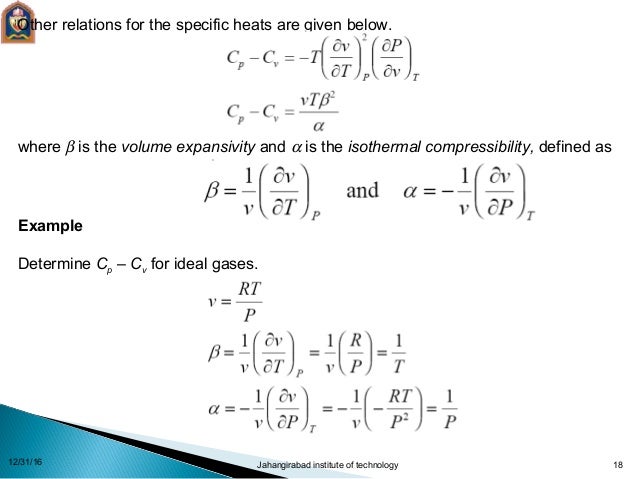
Thermodynamics Relations

Thermodynamic Surface An Overview Sciencedirect Topics

Thermal Engineering 10

Intro Pvt Surface Youtube



